Talent MD
Career Studio
Your go-to source for insights, updates, and expert opinions on healthcaare recruitment, industrytrends, and career advice
Register for Free!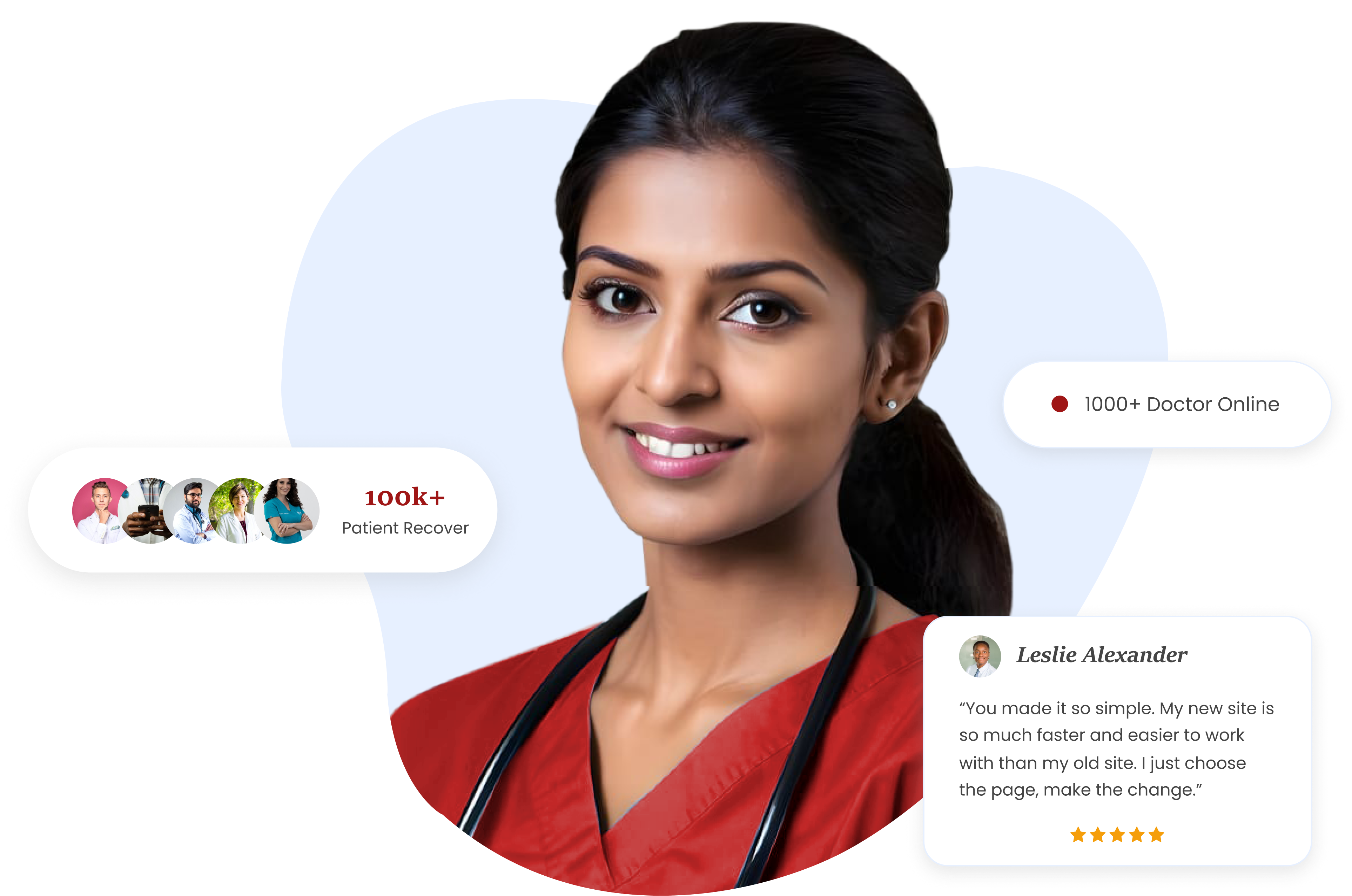
Your go-to source for insights, updates, and expert opinions on healthcaare recruitment, industrytrends, and career advice
Register for Free!
Apr 5th 2024

Interviewing for a Regulatory Affairs Specialist position involves showcasing your expertise in regulatory guidelines, your strategic approach to compliance, and your ability to navigate complex regulatory landscapes. Here are the top 10 interview questions you might face, along with guidance on how to construct effective responses that highlight your qualifications and readiness for the role.
1. Can you describe your experience with regulatory submissions?
Objective:
Assess your hands-on experience with the preparation, submission, and management of regulatory documentation.
Approach:
Provide specific examples of the types of submissions you've managed (e.g., INDs, NDAs, BLAs, MAAs), including the regulatory bodies involved (e.g., FDA, EMA). Highlight your role in the process, the challenges you faced, and how you overcame them.
2. How do you stay updated on changes in regulatory guidelines?
Objective:
Understand your commitment to ongoing education and awareness of the regulatory environment.
Approach:
Discuss the resources you use to stay informed, such as regulatory newsletters, professional associations, conferences, and training sessions. Mention any proactive steps you take to ensure that your knowledge is current and to anticipate changes that could affect your organization.
3. What strategies do you use to ensure compliance in a rapidly changing regulatory landscape?
Objective:
Gauge your ability to adapt strategies to maintain compliance amidst evolving regulations.
Approach:
Share examples of how you've monitored regulatory changes and assessed their impact on ongoing projects. Describe how you've worked with cross-functional teams to implement necessary changes in product development, clinical trials, or marketing strategies.
4. Can you discuss a challenging regulatory project you worked on and how you contributed to its success?
Objective:
Highlight your problem-solving skills and ability to contribute to complex projects.
Approach:
Choose a project that presented significant regulatory challenges, such as achieving approval for a novel therapy or navigating regulatory requirements in multiple jurisdictions. Discuss your role, the specific challenges, the strategies you employed, and the outcome of the project.
5. How do you manage communication with regulatory authorities?
Objective:
Explore your communication skills and ability to foster positive relationships with regulatory bodies.
Approach:
Discuss your experience with direct interactions with regulatory authorities, including preparing for and participating in meetings, responding to queries, and negotiating submission details. Emphasize your ability to communicate clearly, respond promptly, and maintain a collaborative approach.
6. How do you balance business objectives with regulatory requirements?
Objective:
Assess your ability to integrate regulatory considerations into broader business strategies.
Approach:
Provide examples of how you've worked with project teams to understand business goals and explored regulatory pathways that align with those objectives. Highlight how you've contributed to decision-making processes to achieve both compliance and commercial success.
7. Describe your experience with international regulatory submissions.
Objective:
Understand your familiarity with global regulatory environments.
Approach: I
f you have experience, discuss specific submissions you've managed for markets outside your home country, including the challenges of navigating different regulatory landscapes and harmonizing submissions across jurisdictions. If your experience is more limited, discuss how you've prepared to expand your expertise to international regulations.
8. How do you approach the preparation of a risk management plan?
Objective:
Gauge your understanding of risk assessment and mitigation in the regulatory context.
Approach:
Explain your process for identifying potential regulatory risks associated with a product or project, assessing the likelihood and impact of those risks, and developing strategies to mitigate them. Include examples of how you've implemented or contributed to risk management plans in the past.
9. What role do you believe regulatory affairs specialists play in product development?
Objective:
Explore your understanding of the strategic importance of regulatory affairs in product lifecycle management.
Approach:
Discuss how regulatory affairs specialists contribute to product development by providing strategic guidance on regulatory requirements, facilitating effective communication with regulatory bodies, and ensuring that development activities align with compliance objectives from an early stage.
10. Why are you interested in working for our company as a Regulatory Affairs Specialist?
Objective:
Demonstrate your motivation and fit for the organization.
Approach:
Research the company's product portfolio, pipeline, regulatory challenges, and corporate culture. Discuss how your experience and skills align with the company's needs and how you are excited about the opportunity to contribute to its success.
When answering these questions, it's crucial to demonstrate not only your technical knowledge and experience but also your strategic thinking, communication skills, and ability to collaborate effectively across functions. Tailor your responses to reflect the specific requirements of the role and the organization's focus, whether it's pharmaceuticals, biotechnology, medical devices, or another regulated industry.
Role Codes
Resume Tips
Interview Questions
Hospital Departments
Hospital Administration
Recruitment Trends
Career Development
Job Search Strategies
Healthcare Innovations
Interview Preparation
Career Tips
Employee Benefits
Workplace Culture
Freelance Opportunities
Remote work
Freshers in Healthcare