Talent MD
Career Studio
Your go-to source for insights, updates, and expert opinions on healthcaare recruitment, industrytrends, and career advice
Register for Free!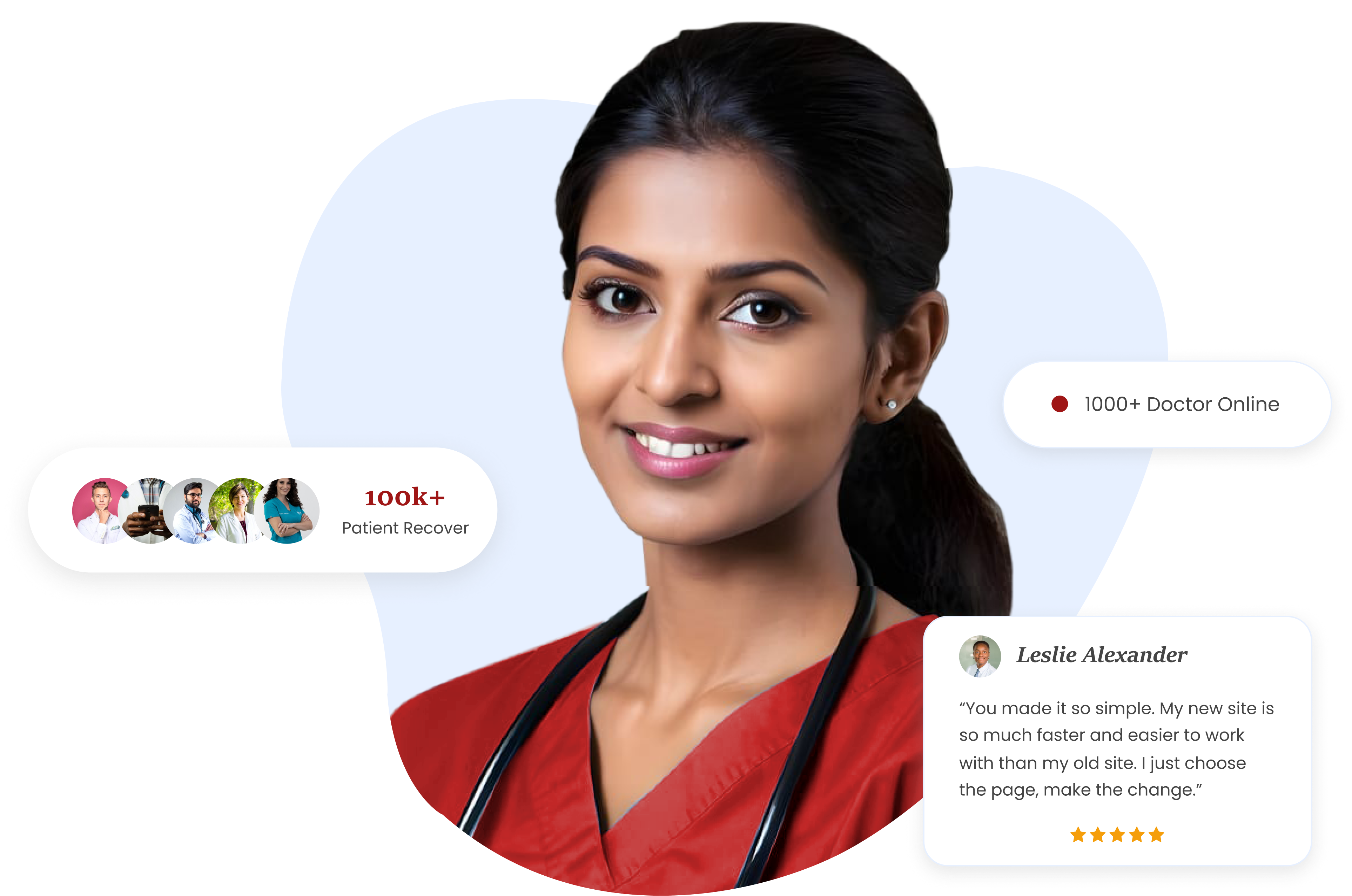
Your go-to source for insights, updates, and expert opinions on healthcaare recruitment, industrytrends, and career advice
Register for Free!
Mar 30th 2024

Interviewing for a position focused on enhancing clinical trials efficiency through digital platforms requires a blend of knowledge in clinical research, digital technology, and project management. Here are the top 10 interview questions you might face, along with objectives for each question and suggestions on how to effectively answer them.
1. Can you describe your experience with digital platforms in clinical trials?
Objective:
Assess hands-on experience with digital technologies in the context of clinical research.
Suggestion:
Discuss specific platforms you've used, such as electronic data capture (EDC), patient management systems, or remote monitoring tools. Highlight how these technologies improved trial efficiency, data quality, or participant engagement in your past projects.
2. How do digital platforms improve patient recruitment and retention in clinical trials?
Objective:
Gauge understanding of the benefits of digital tools in enhancing participant engagement.
Suggestion:
Explain how digital platforms facilitate wider outreach through social media and online communities, enable targeted advertising, and provide convenient, remote consent processes. Also, mention how these tools support continuous engagement and feedback, improving retention rates.
3. What are the key challenges in integrating digital platforms into clinical trials and how would you address them?
Objective:
Understand awareness of and solutions to potential obstacles in digital adoption.
Suggestion:
Discuss issues such as data security, regulatory compliance, user training, and interoperability between systems. Offer strategies like adopting platforms with robust encryption standards, ensuring compliance with regulations like GDPR or HIPAA, providing comprehensive training programs, and choosing platforms with API integration capabilities.
4. How do you ensure data integrity and compliance when using digital platforms for clinical trials?
Objective:
Evaluate knowledge of data management principles and regulatory standards.
Suggestion:
Describe measures such as using platforms that comply with 21 CFR Part 11, implementing data validation processes, conducting regular audits, and ensuring that data is securely backed up. Mention any experience you have with regulatory inspections or audits related to digital data management.
5. Can you give an example of a successful clinical trial you managed or participated in that utilized digital platforms?
Objective:
Seek evidence of successful application and outcomes of digital technologies in trials.
Suggestion:
Share a specific case where digital platforms played a crucial role in the trial's success, focusing on the objectives, the digital tools utilized, the challenges overcome, and the outcomes achieved. Highlight any innovations or efficiencies gained through digital implementation.
6. How do you stay updated with the latest advancements in digital health technologies relevant to clinical trials?
Objective:
Assess commitment to continuous learning and professional development.
Suggestion:
Mention specific journals, conferences, professional networks, or online courses focused on digital health, clinical research technology, or biostatistics. Discuss how you apply this continuous learning to your work.
7. What is your approach to training and supporting clinical trial staff in using new digital platforms?
Objective:
Understand strategies for ensuring team competency in digital tools.
Suggestion:
Outline a structured training program that includes hands-on workshops, regular updates on new features, access to online resources, and ongoing support mechanisms. Emphasize the importance of building a digital culture that embraces innovation.
8. In your opinion, what is the future of digital platforms in clinical trials?
Objective:
Gauge insight into future trends and innovations in clinical trial management.
Suggestion:
Discuss emerging technologies such as AI and machine learning for predictive analytics, blockchain for secure data sharing, and VR for patient education. Highlight the potential for these technologies to further streamline trial processes, enhance data analysis, and improve participant engagement.
9. How do you balance innovation with regulatory compliance when implementing digital solutions in clinical trials?
Objective:
Assess ability to innovate within the confines of regulatory frameworks.
Suggestion:
Talk about the importance of early engagement with regulatory bodies, leveraging regulatory guidance on digital health technologies, and participating in pilot programs or sandbox environments to test new solutions while ensuring compliance.
10. What metrics do you use to evaluate the success of digital platform integration in clinical trials?
Objective:
Evaluate understanding of key performance indicators (KPIs) for digital implementation.
Suggestion:
Mention metrics such as recruitment rates, retention rates, data entry errors, time to data lock, and participant satisfaction. Discuss how you use these metrics to continually assess and improve the effectiveness of digital tools in trial processes.
In answering these questions, highlight your expertise in digital technologies, your strategic approach to overcoming challenges, and your vision for the future of digital platforms in clinical trials. Demonstrating a track record of successful implementation and a commitment to innovation and continuous improvement will position you as a strong candidate.