Talent MD
Career Studio
Your go-to source for insights, updates, and expert opinions on healthcaare recruitment, industrytrends, and career advice
Register for Free!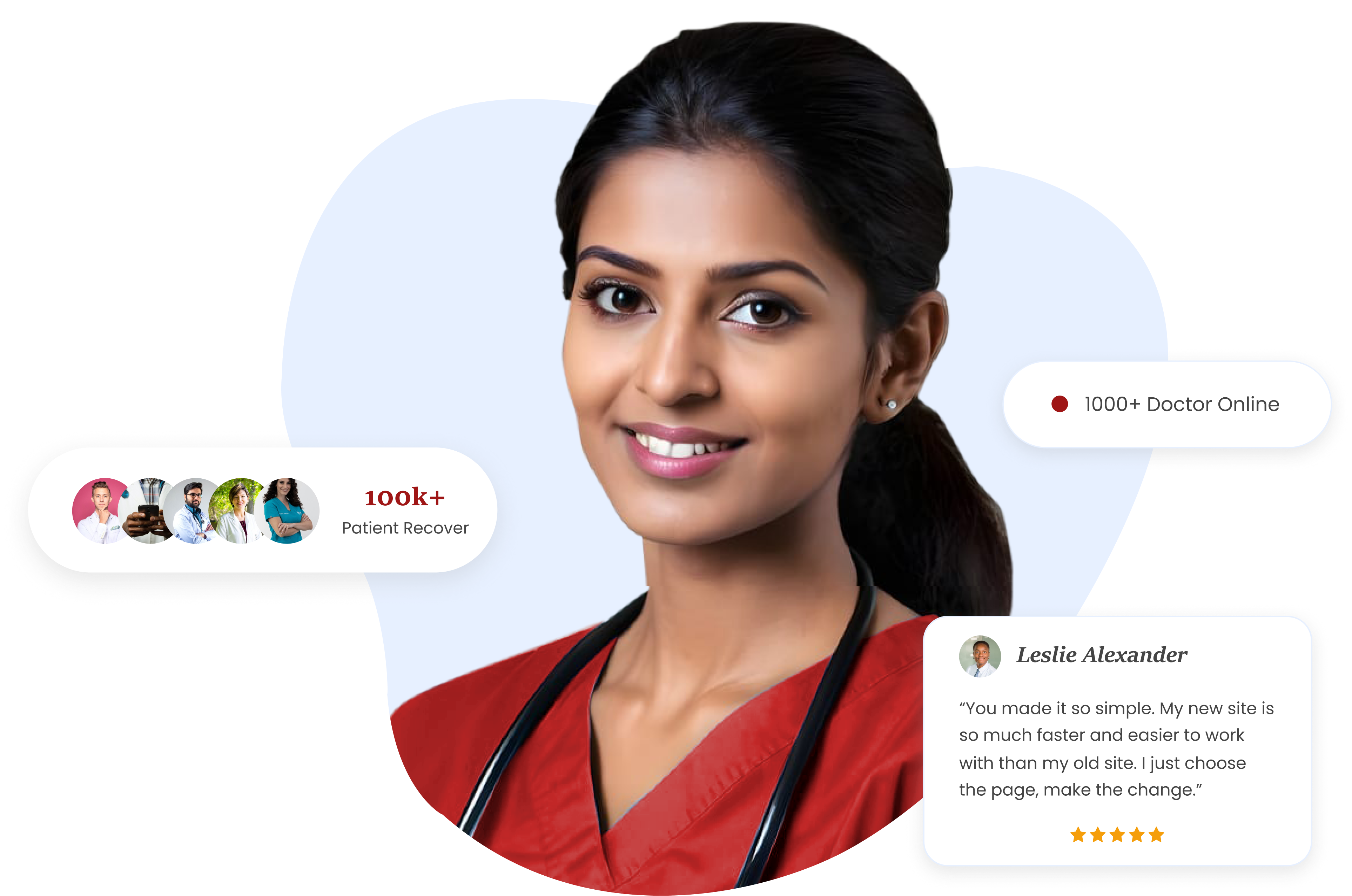
Your go-to source for insights, updates, and expert opinions on healthcaare recruitment, industrytrends, and career advice
Register for Free!
Mar 28th 2024

Mastering the top interview questions for roles focused on balancing compliance and innovation in hospital administration requires a nuanced understanding of the regulatory landscape as well as a forward-thinking approach to healthcare management. Here's how to prepare for ten critical interview questions, with objectives for each answer and suggestions on how to effectively respond.
1. How do you keep abreast of changes in healthcare regulations and ensure your facility remains compliant?
Objective:
Demonstrate your commitment to continuous learning and adherence to legal standards.
Suggestion:
Discuss your methods for staying updated, such as subscribing to healthcare regulation newsletters, attending workshops, and participating in professional networks. Highlight your proactive approach to implementing changes in policies or practices to maintain compliance.
2. Describe an innovative project you implemented in your last role. How did you navigate the regulatory challenges?
Objective:
Show your ability to innovate within the constraints of healthcare regulations.
Suggestion:
Provide a specific example that highlights your creative problem-solving skills, your collaboration with regulatory bodies or internal compliance teams, and the impact of the innovation on patient care or operational efficiency.
3. What strategies do you use to balance innovation with regulatory compliance?
Objective:
Illustrate your strategic approach to maintaining this balance.
Suggestion:
Talk about your methodology for risk assessment, how you prioritize projects that offer significant benefits with manageable compliance challenges, and the way you involve compliance teams early in the planning process.
4. How do you encourage a culture of innovation while ensuring compliance among your team?
Objective:
Demonstrate your leadership in promoting both innovation and compliance.
Suggestion:
Discuss how you lead by example, encourage open communication, provide training and resources related to compliance, and recognize innovative ideas that successfully navigate regulatory requirements.
5. Can you give an example of a time when you had to modify an innovative project to ensure compliance?
Objective:
Show your flexibility and commitment to compliance without stifling innovation.
Suggestion:
Choose an example where adjustments to an innovative approach were necessary and explain how you collaborated with your team and compliance officers to alter the project while still achieving valuable outcomes.
6. How do you assess the potential compliance risks of new healthcare technologies or practices?
Objective:
Highlight your analytical and forward-thinking approach to risk assessment.
Suggestion:
Describe the tools and frameworks you use to evaluate new technologies or practices, including conducting a comprehensive risk analysis, consulting with legal experts, and considering the ethical implications for patient care.
7. What is your process for integrating new regulations into existing operational practices?
Objective:
Show your systematic approach to operational compliance.
Suggestion:
Outline the steps you take, such as reviewing new regulations for impact, updating policies and procedures, training staff on new requirements, and monitoring implementation to ensure compliance.
8. How do you prioritize which innovations to pursue in light of regulatory and budgetary constraints?
Objective:
Demonstrate your ability to make strategic decisions that align with organizational goals and limitations.
Suggestion:
Discuss how you evaluate the potential ROI of innovations, consider the regulatory landscape, and align projects with the strategic priorities of the healthcare facility, including patient care quality and safety.
9. Describe a scenario where you successfully advocated for a regulatory change to facilitate innovation at your facility.
Objective:
Showcase your advocacy skills and ability to influence regulatory bodies.
Suggestion:
Provide a detailed account of your efforts to engage with regulatory authorities, including the preparation of evidence-based arguments, coalition-building with industry partners, and the positive outcomes achieved through regulatory change.
10. How do you ensure that long-term innovation projects remain compliant with evolving healthcare regulations?
Objective:
Illustrate your long-term planning and adaptability.
Suggestion:
Explain your approach to monitoring regulatory changes that might affect ongoing projects, including periodic reviews of project compliance, adjustments to project plans as necessary, and maintaining open lines of communication with regulatory bodies.
In preparing your answers, emphasize your proactive stance on compliance, your innovative mindset, and your leadership qualities. Providing concrete examples will help illustrate your effectiveness in navigating the complex landscape of healthcare administration, balancing the need for regulatory compliance with the drive for innovation.